Battery Types Explained: Basics You Should Know
- 30 June 2025
- 20 views
- No Comments
Battery Types Explained: Basics You Should Know
What is the battery?
The answer to this question is as simple as a device to store the energy. Batteries do not provide energy. Batteries are electronic devices that store energy and deliver it to the circuit when needed. Their ability to store and release energy depends on the chemical reactions inside. Even a small change in these chemicals can significantly affect how the battery stores or releases energy. Rechargeable batteries repeat the process of chemicals to charge or discharge the battery. Since batteries are not 100% efficient, some energy loss occurs due to the charge/discharge process, and it’s
different for various types of batteries and can differ from 5%-25%
Different principles affect batteries. One of them is the temperature. The reason behind the increase in battery temperature is internal resistance. Internal resistance accounts for energy. The performance of a battery is another principle. The performance of a battery actually depends on how the batteries are used. Various types of batteries represent different performances in electronic circuits. Lead-acid batteries, for example, demonstrate 85-95% efficiency while alkaline and Ni-Cad batteries are about 65% efficient. some deep-cycle AGM (Absorbed Glass Mat) batteries can achieve up to 98% efficiency under ideal conditions, but this is rare. In most cases, battery setups experience a 10-20% energy loss. in this article, we will explore different types of batteries and explain their key characteristics.
How to distinguish different types of batteries:
Several characteristics help differentiate one battery from another, with capacity and depth of discharge (DoD) being two of the most important ranking factors.
Voltage: The voltage of a battery is a fundamental characteristic that is determined by its chemicals. Different batteries come in different voltages, such as 2V, 3.7V, 6V, and 12V.
Capacity: Capacity refers to the amount of charge a battery can store. It represents the maximum energy the battery can deliver under specific conditions. However, the nominal capacity of the battery can change noticeably in different conditions. Various factors, including depth of charge/discharge, age, and climate conditions, can affect the nominal capacity of the battery.
Depth of Discharge: Depth of Discharge: When a battery operates in a system, it supplies a portion of its stored energy. We measure this portion using the depth of discharge. Many batteries cannot be fully discharged without risking damage. For example, if the manufacturer states a DoD of 30%, the system should only use up to 30% of the battery’s total capacity.
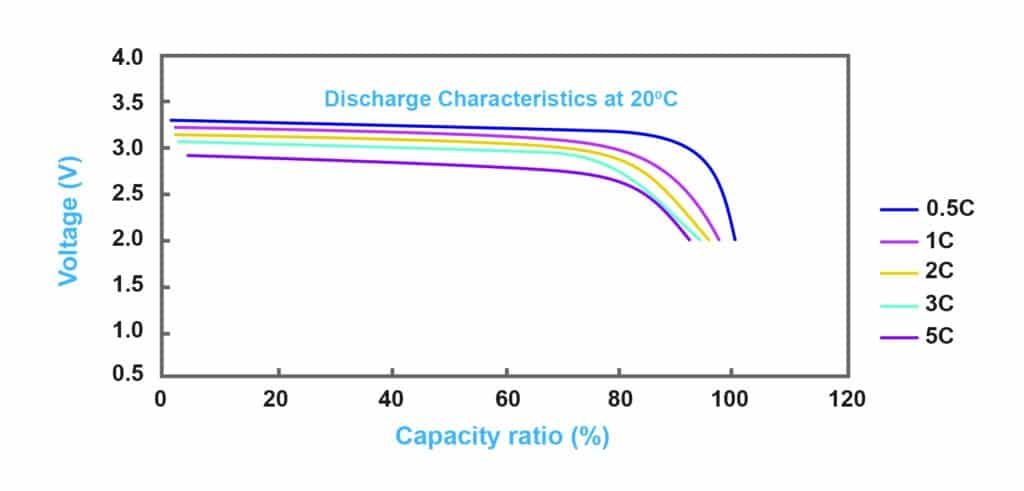
Batteries follow an international technical standard known as the C-rate (or charging ratio). This standard is expressed in terms such as 0.1c, 0.2c, or c20, and it indicates how fast a battery charges or discharges relative to its rated capacity. This means that, for example, if the battery is 100 AH, in 1C conditions, the charging or discharging current is 100A. Also, for C20, C20 stands for 0.05c charging ratio, so a 100AH battery will be empty after 20 hours in C20 standard.
Types of Batteries
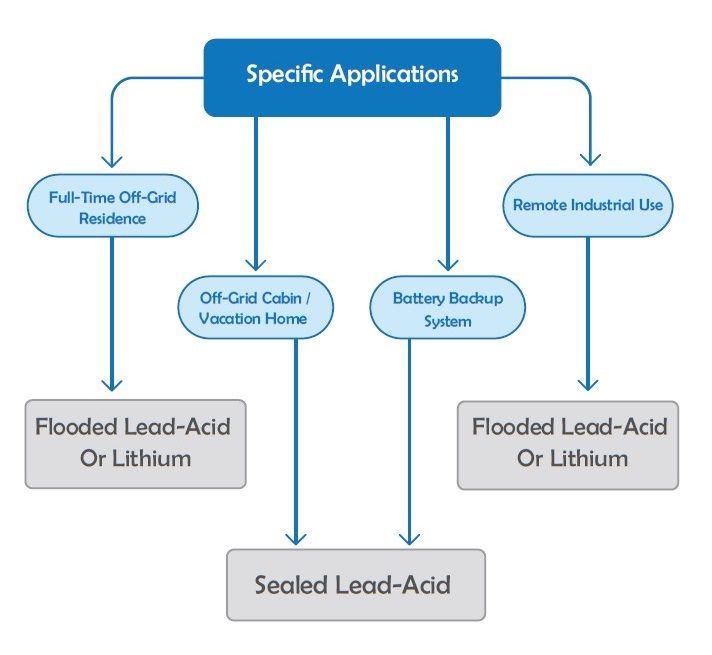
Different applications require different types of batteries. We can categorize batteries based on two key principles: their intended application and their internal construction. For large systems, common applications are usually automotive, marine, and deep-cycle. Photovoltaic systems, backup power, traction, and boat batteries are specific areas for deep-cycle batteries. According to construction, batteries are classified into flooded, gelled, and sealed AGM batteries. Depending on the energy sector, one might use different kinds of batteries. AGM, VRLA, Lead Acid, lithium and etc. might look confusing, so in this article, we will try to make it easy to understand the most common types of batteries and the differences between them.
Automotive (Starting) Batteries
This type of battery is employed to start/run the engines, that’s why they’re also called starting batteries. Engine starters are in need of a very large amount of current for a short time. Starting batteries use many thin plates to maximize surface area, which allows them to deliver a higher starting current. While these kinds of batteries perform well as sudden current providers, they are not proper for deep-cycle applications. For example, in PV applications, automotive batteries with sponge-like plates degrade quickly. The plates break down and settle at the bottom of the cell, limiting their lifespan to fewer than 150 cycles. However, these batteries perform well and last much longer in standard engine-starting applications.
Deep-Cycle Batteries
These batteries use thicker plates made from solid lead. This will lead to lower surfaces and, accordingly, less instant power, unlike the starting batteries. Manufacturers design these batteries specifically for deep cycling applications. Thick plates reduce the total surface area, which results in a battery that delivers lower max current but allows a deeper state of charge. Deep-cycle batteries typically run at discharges down to 50% of their nominal capacity, making them ideal for applications that need a steady power supply over long periods. Users can discharge them as low as 20%, but it’s better to avoid dropping below 50% to extend battery life. To keep the batteries at full capacity, users should fully recharge them at least once a month. If they don’t, the capacity will gradually drop over time. To maintain their full capacity, it’s also important to fully recharge them at least once a month. Failing to do so will gradually reduce their capacity over time. Deep-cycle batteries are available in various types, such as AGM or GEL batteries.
Marine Batteries
Marine batteries combine features of both starting and deep-cycle batteries, which is why people often call them “hybrid” batteries. Manufacturers build these batteries using coarser and heavier spongy plates so that the batteries can serve both starting and deep-cycle functions.
What Is Inside A Battery?
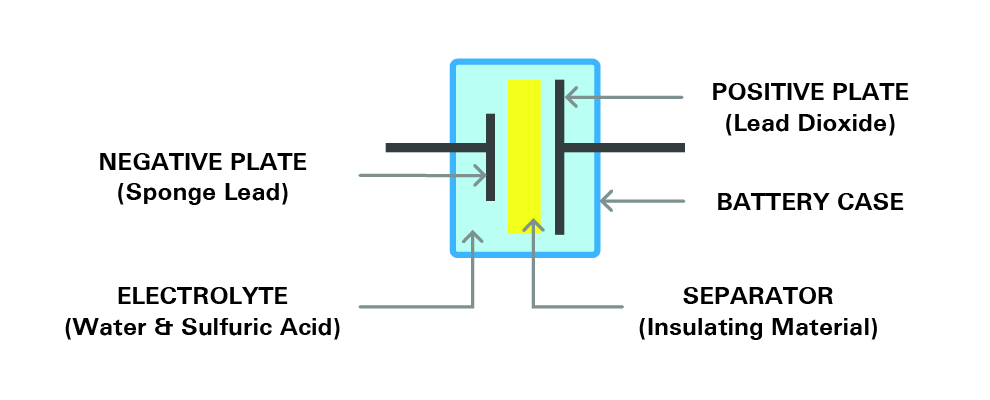
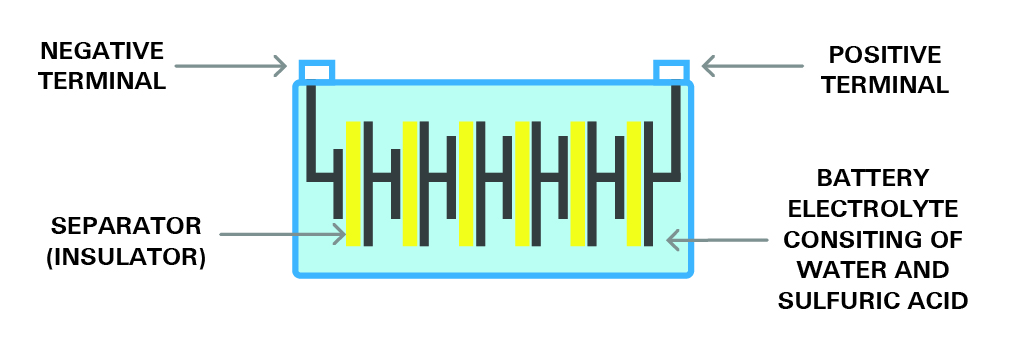
This section explains the mechanism and construction of a battery. Most of the available industrial rechargeable batteries are lead-acid batteries. To understand the internal structure of a battery, we take the lead-acid battery as the basic example in this article. A battery consists of several single cells, each producing approximately 2.1 volts, connected in series.
For example, a six-volt battery has three single cells, and a twelve-volt battery has six single cells in series. Each cell consists of two lead plates. Each cell includes a positive plate coated with lead dioxide and a negative plate made of sponge lead. There is also a separator between. The plates sit in an electrolyte made of water and sulfuric acid, all enclosed within a sealed container. Each cell is capable of storing 2.1 volts. The image below shows the structure of a single cell.
A typical 12-volt battery uses six 2.1-volt cells connected in series. The diagram below illustrates this configuration. When fully charged, the battery produces a total voltage of 12.6 volts.
The lifetime of a Battery
How you use a battery greatly affects its lifetime. Maintenance, charging process and climate conditions are also important factors in the life of a battery. A battery can die from severe overcharging in less than a year, while suitable care and maintenance can drive a battery under heavy service over long years. Usually, lead-acid batteries are prone to danger due to the higher rates of discharge. If you deeply discharge a lead-acid battery (beyond 80%), it won’t regain its full capacity during the next charge. In contrast, lithium-ion batteries don’t suffer from memory effects, so you can discharge them deeply without damaging them.
Temperature is another principle in determining a battery’s life. Batteries function best at room temperature. Operating a battery at higher temperatures improves performance, but prolonged exposure will shorten its life. All batteries achieve optimum lifetime if used at 20°C. In increased temperatures, the cycle life is reduced by 20%, 30%, 50% and even higher.
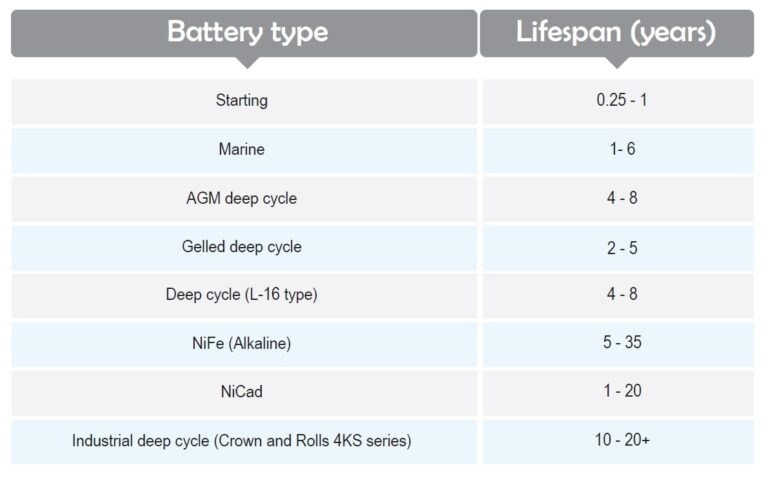
Here are the typical expectations of various types of batteries in normal maintenance conditions:
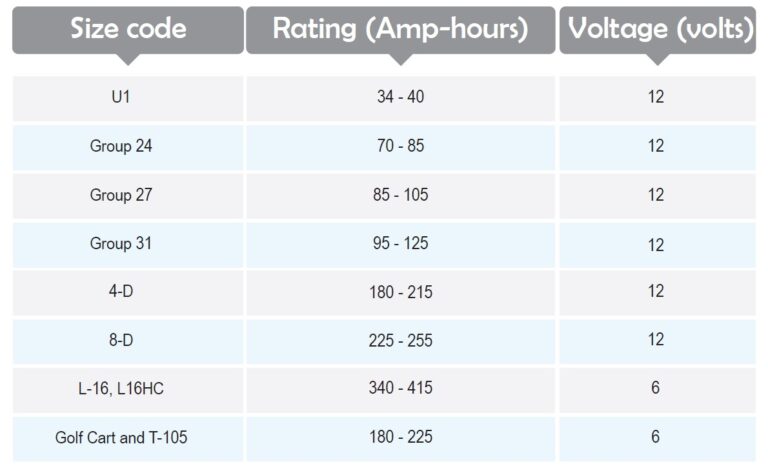
Battery Size Codes
Batteries are different in size, so a standard code system has been developed to categorize them. However, many batteries do not belong to any of these codes, but it is a nice reference to adjust the physical size and terminal placement of the batteries. Battery ratings are typically expressed in amp-hours (Ah), which indicate how much current a battery can supply over a one-hour period. For example, if you use a 60-amp battery over a 15-minute cycle, the usage equals 60 × 0.25 = 15 amp-hours.
Photovoltaic Batteries
Batteries play a crucial role in every off-grid solar system. People usually need more energy in the evening, but solar panels generate most of their energy during the day. By using a battery, users can store solar power generated during the day and use it when they actually need it.
In this section, we will examine the batteries used in solar systems and compare their advantages and disadvantages. This information will help readers choose the right battery for their solar setup. Most solar systems use batteries from the deep-cycle category, which supply energy for renewable energy systems. Deep-cycle batteries handle repeated charging and deep discharging, which sets them apart from other types, like automobile batteries.
Manufacturers commonly use four main types of batteries in solar systems and other renewable energy setups. We will explain these types in detail in this article. In this article, these types will be discussed in detail.
1) Lead-Acid
Sealed lead-acid batteries are a group of this family that is maintenance-free. Several types of sealed lead-acid have emerged, and the most common are GEL, also known as valve-regulated lead-acid (VRLA), and absorbent glass mat (AGM). Electrodes of this type are grids of metallic lead-containing lead oxides that change during the charge/discharge process. They are bulky and occupy more space than other types of batteries, so they can’t be considered a suitable choice for “on-grid” applications. Before recent achievements, lead-acid batteries were the only practical choice to store solar energy. The combination of AGM technology and lead-acid batteries makes them one of the best batteries to employ in solar systems. AGM batteries differ from gel batteries in that they do not use gelled electrolytes. Instead, they use a “recombinant gas absorbed electrolyte” design, which reduces water loss by up to 98%. Thanks to their low internal resistance, they outperform many other battery types in efficiency. Their self-discharge rate is 3–10 times lower than that of gel batteries and 5–50 times lower than traditional flooded lead-acid batteries.
The development of AGM sealed battery technology began in 1980 and by 1985, it was adopted for military aircraft to evaluate the reliability of this design under demanding conditions.
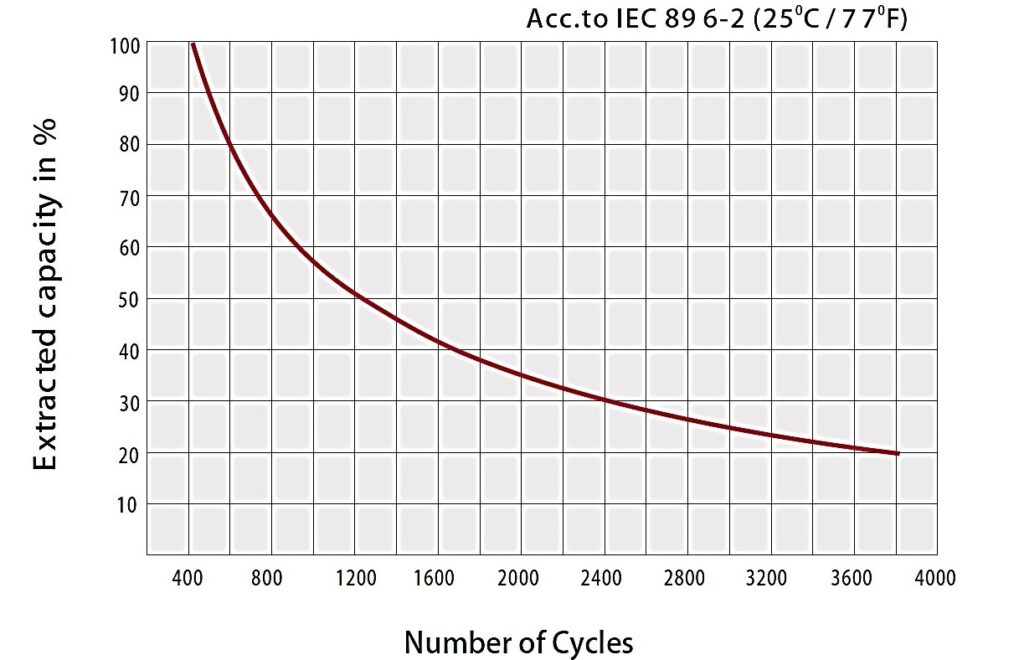
Lead-acid batteries benefit from a long life, which is highly dependent on the type of battery—for example, industrial batteries can live up to 20 years while standard ones live for 3–5 years. It should be noted that this lifespan is a matter of careful maintenance. This type of battery also suffers from practical downsides. Figure 8 demonstrates the capacity of lead-acid batteries after deep cycles. It can be noted that the battery decreases to 20% capacity for 3800 cycles.
2) Lithium-Ion Batteries
Lithium batteries have many advantages over traditional battery types, including long life cycles and higher charge/discharge rates. Lithium batteries used in solar systems typically rely on Lithium Iron Phosphate (LiFePO₄) as their chemical base. This compound offers excellent thermal stability, high current capacity, and a long lifecycle. Unlike flooded lead-acid and other battery chemistries, lithium batteries do not vent dangerous gases such as hydrogen or oxygen, so they are less prone to the risk of explosion. Each lithium battery cell has a nominal voltage of 3.6V, compared to 2V per cell in lead-acid batteries. As a result, lithium batteries require fewer cells to achieve the same system voltage. Despite this difference, both types of batteries have similar charging voltages, which makes lithium batteries a convenient replacement for lead-acid systems. Lithium batteries don’t require regular full charging, while lead-acid batteries can experience plate degradation if they start discharging before reaching a full charge. Another important factor is performance efficiency, where lead-acid batteries represent 80% efficiency during their life cycle, while this amount can reach 95–98% by lithium-ion batteries. Currently, the primary drawback of lithium batteries is their relatively higher price compared to lead-acid options.
3) NICAD (Nickel Cadmium)
In this type of battery, the positive electrode uses nickel oxide, while the negative electrode contains cadmium. Alkaline batteries work well for emergency or standby systems but are not suitable for daily cycling, so they are not recommended for use in solar energy systems. NICADs are very expensive, and cadmium is a hazardous material for human beings, and they also suffer from low efficiency (65–80%).
Solar systems also use other battery types, such as Sodium Nickel Chloride, Nickel-Iron (NiFe), and flow batteries. These technologies offer relatively long lifecycles—typically around 3,000 to 4,000 cycles—but their high costs limit their use. In contrast, lead-acid and lithium batteries are more cost-effective and remain the preferred choices over alkaline and flow batteries in most solar applications. Besides the low life cycle, low efficiency is another disadvantage of alkaline batteries.
Closer Look at Lead-Acid and Lithium Batteries
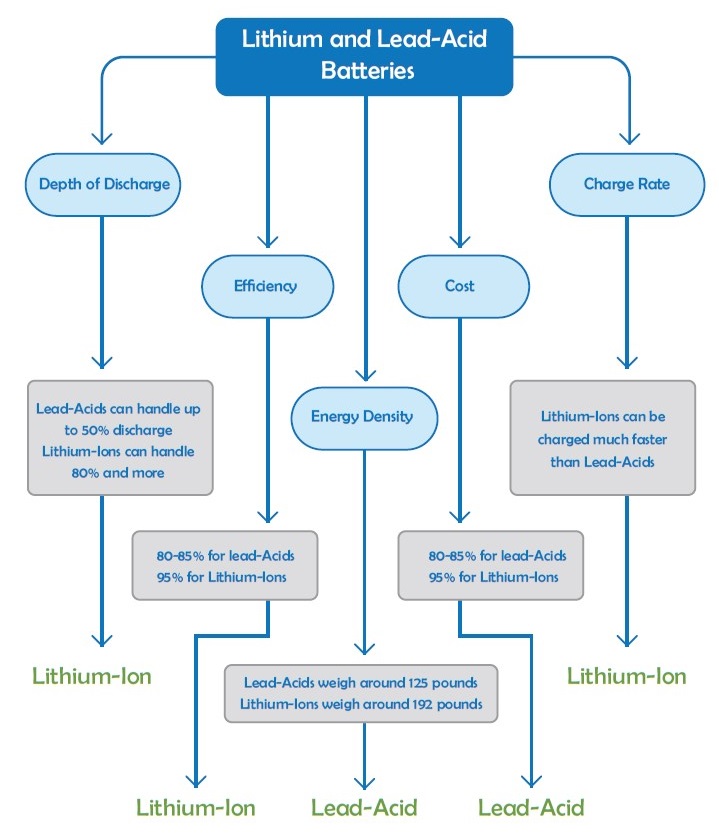
In this section, we will take a closer look at lead-acid and lithium batteries, as they are the most suitable options for use in solar and backup power systems. Their life cycle, cost, and efficiency make them suitable to these kinds of applications. Here, we will introduce various types of lead-acid batteries. The cells of lead-acid batteries are generally 2V, so for 12V, we need six cells in series.
Flooded Lead-Acid Batteries
This type is widely used in the automotive industry and is considered one of the most cost-effective options for solar systems. Normal flooded batteries require extra care and regular maintenance for proper watering, equalizing charges and keeping the terminals clean.
Users must handle flooded batteries with care, as they are subject to specific transportation regulations.
Sealed Lead-Acid Batteries
The thickness of the battery plates determines the ability and application of the battery. SLAs do not tend to be sulfate or as wet cell batteries, so they’re the safest type of lead-acid batteries. Sealed Lead-Acid (SLA) batteries are divided into two main types: AGM and Gel cells. Manufacturers typically package them in plastic containers, and they are commonly used in small UPS systems, emergency lighting, and wheelchairs. The low price, reliability and low maintenance are the prominent features of these batteries. They are recommended in hospitals, retirement homes, internet hubs, banks and etc. However, their performance declines in low-temperature climates and becomes significantly worse when temperatures drop below 0°C.
AGM Sealed Lead-Acid Battery
Absorbed Glass Matt (AGM) process is prior to traditional flooded technology. These batteries can charge faster than other types due to their lower internal resistance. AGM batteries deliver higher capacity in a smaller form factor and can be shipped using standard transportation methods. The AGM suspends the electrolyte in a designed glass-mat, which leads to a faster-charging process and instant high load currents, so they are suitable for starters of motorcycles, hybrid-cars, and large solar systems.
With cycling, the capacity of AGM decreases gradually; gel, on the other hand, demonstrates high performance for a longer time but involves a sudden performance decrease at the end of its life. AGM batteries cost less than gel batteries, while they are more expensive than flooded ones. Sealed lead-acid batteries prevent gas generation through voltage regulation. Water depletion, dry-out, and gassing are results of overcharging the battery. VRLA (valve-regulated lead-acid) batteries are optimized to operate at 25°C. For every 8°C increase above this temperature, their lifespan is reduced by half.
Gel Sealed Lead Acid
Gel VRLA batteries contain a gelled electrolyte that separates them from AGM counterparts. Mixing sulfuric acid with silica fume creates a gel-like substance, which makes these batteries maintenance-free. This battery type supports standard shipping methods and requires no special handling. GEL batteries are resistant to higher temperatures, shock, and vibration. Over-discharging can cause serious damage to flooded and some AGM batteries, while gel batteries are more resistant to issues related to overcharging and deep discharging. They are the proper choice for constant current applications. Gel batteries perform well at low-temperature conditions, which makes them superior to sealed batteries.
Gel batteries are more expensive than AGM batteries and offer a very low self-discharge rate—around 1% per month. However, they require specific gel-compatible chargers and should always be used with them.
Lithium Batteries
Lithium-ion batteries are becoming increasingly accepted over lead-acid batteries because of their various advantages. Lithium-ion batteries have become the standard for use in PV systems. Based on their lifespan, lithium-ion batteries typically last around 15 years—about five to ten years longer than lead-acid batteries. On the other hand, ventilation is not necessary for lithium batteries. And the most important advantage of the lithium-ion batteries is that they discharge up to 100%. However, the deeper discharge will reduce their lifespan.
Lithium batteries offer two important protection features.
First, the battery automatically disconnects both input and output when its voltage exceeds the maximum protection limit. Similarly, it disconnects the output when the voltage drops too low, preventing damage to the system.
Second, the battery responds to extreme temperature conditions. If it overheats, the system cuts off both input and output. When the temperature drops below 0°C, it disables input charging, and below -20°C, it also disables output to protect the battery.
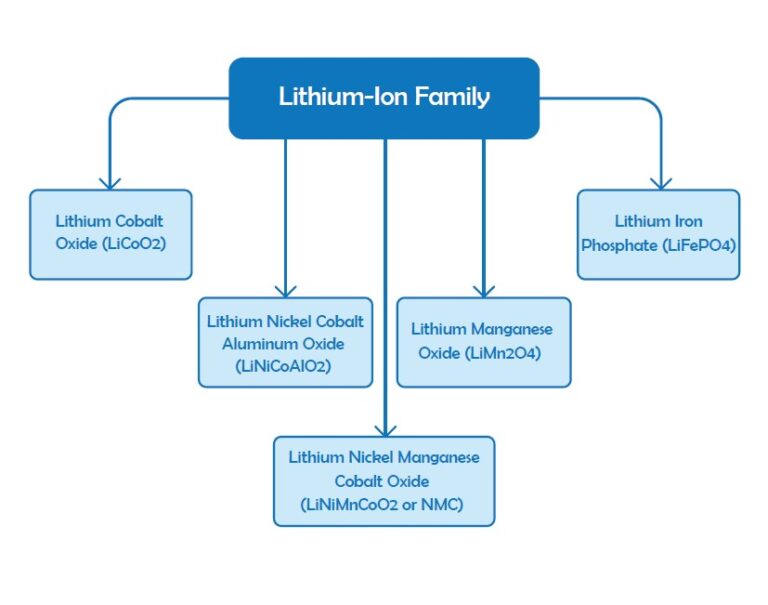
The term “lithium-ion” stands for a family of batteries with noticeable similarities but different chemistries such as Li-cobalt, Li-Manganese, NMC and Li-Aluminum, Li-Phosphate, Li-Titanate, and Lithium Iron Phosphate. As mentioned in the previous sections Lithium Iron Phosphate (LiFePO₄) is of the common choices for solar applications because of the long lifetime, deeper cycles, maintenance-free and no venting characteristics. Li-Manganese, NMC and Li-Aluminum and Li-Phosphate are employed in portable applications because of their higher capacity. Li-Titanate and Lithium Iron Phosphate have lower voltages and have less capacity, but are very enduring. The setup of lithium batteries will be expensive than lead-acids but their efficiency and long lifespan can be a good reason to utilize them in PV systems. Lithium batteries provide 3.6V voltage for each cell which makes them as a proper choice for mobile devices to reduce the cost of multi-cell designs. These types of batteries require regular protection and they are among the expensive batteries.